Sub-EFFECT Background
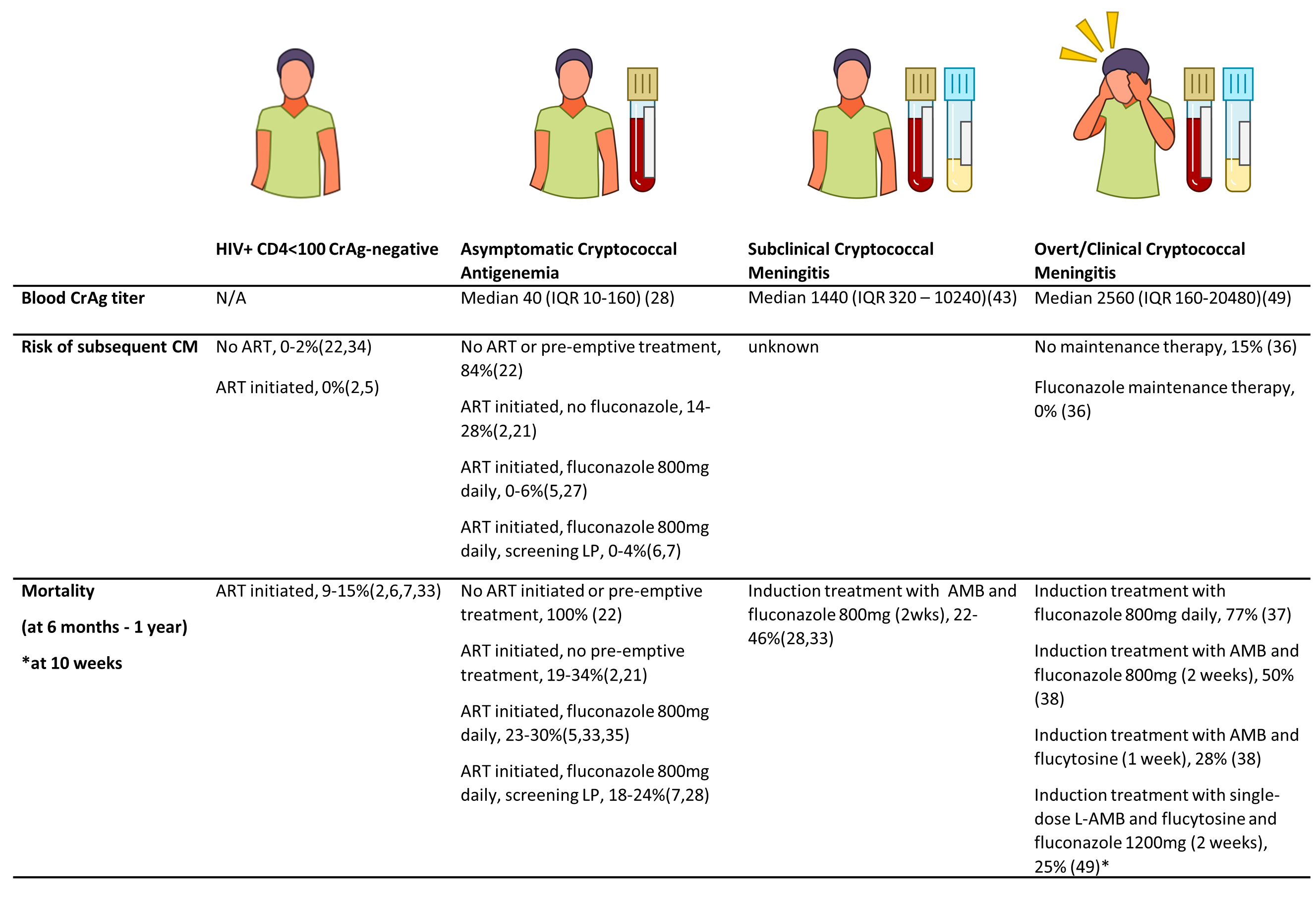
The sub-EFFECT Study is an observational cohort study of patients with subclinical cryptococcal meningitis, who are excluded from the EFFECT trial (Efficacy of Flucytosine and Fluconazole as Early Cryptococcosis Treatment, (ISRCTN30579828). EFFECT is a randomised control trial, which aims to compare the efficacy of an oral combination of fluconazole plus flucytosine with fluconazole alone (the current recommended treatment) in reducing all-cause mortality in patients living with HIV with CD4 counts of <100 cells/µL and asymptomatic cryptococcal antigenaemia (blood CrAg-positive patients) in Tanzania and South Africa. There is now a considerable body of evidence revealing that CrAg-positive patients have substantially higher mortality compared to CrAg-negative patients with similar CD4 counts, despite pre-emptive treatment with fluconazole (25%-33% mortality vs. 9%-15% in different studies)(1–3). A recent study investigating cause of death among asymptomatic CrAg-positive patients in South Africa observed cryptococcal-related deaths following fluconazole monotherapy indicating that this is inadequate pre-emptive treatment(2).
One possible explanation for excess mortality among CrAg-positive patients, is that despite an absence of meningitis signs and symptoms, up to 40% have subclinical cryptococcal meningitis (CM) (cerebrospinal fluid (CSF) positive for Cryptococcus by culture, india ink microscopy and/or CrAg test), at the time of the screening blood test, with an increased risk in those with higher blood CrAg titres(3,4) (Figure 1). Recent clinical guidelines therefore recommend performing lumbar punctures (LPs) in all blood CrAg-positive patients, irrespective of symptoms or signs of meningitis(5). In accordance with these guidelines, all patients eligible for the EFFECT study will be offered a LP, and will be excluded if Cryptococcus is detected in the CSF. These patients will then be treated routinely for CM by their usual practitioners and invited to participate in the sub-EFFECT observational cohort study at participating sites.
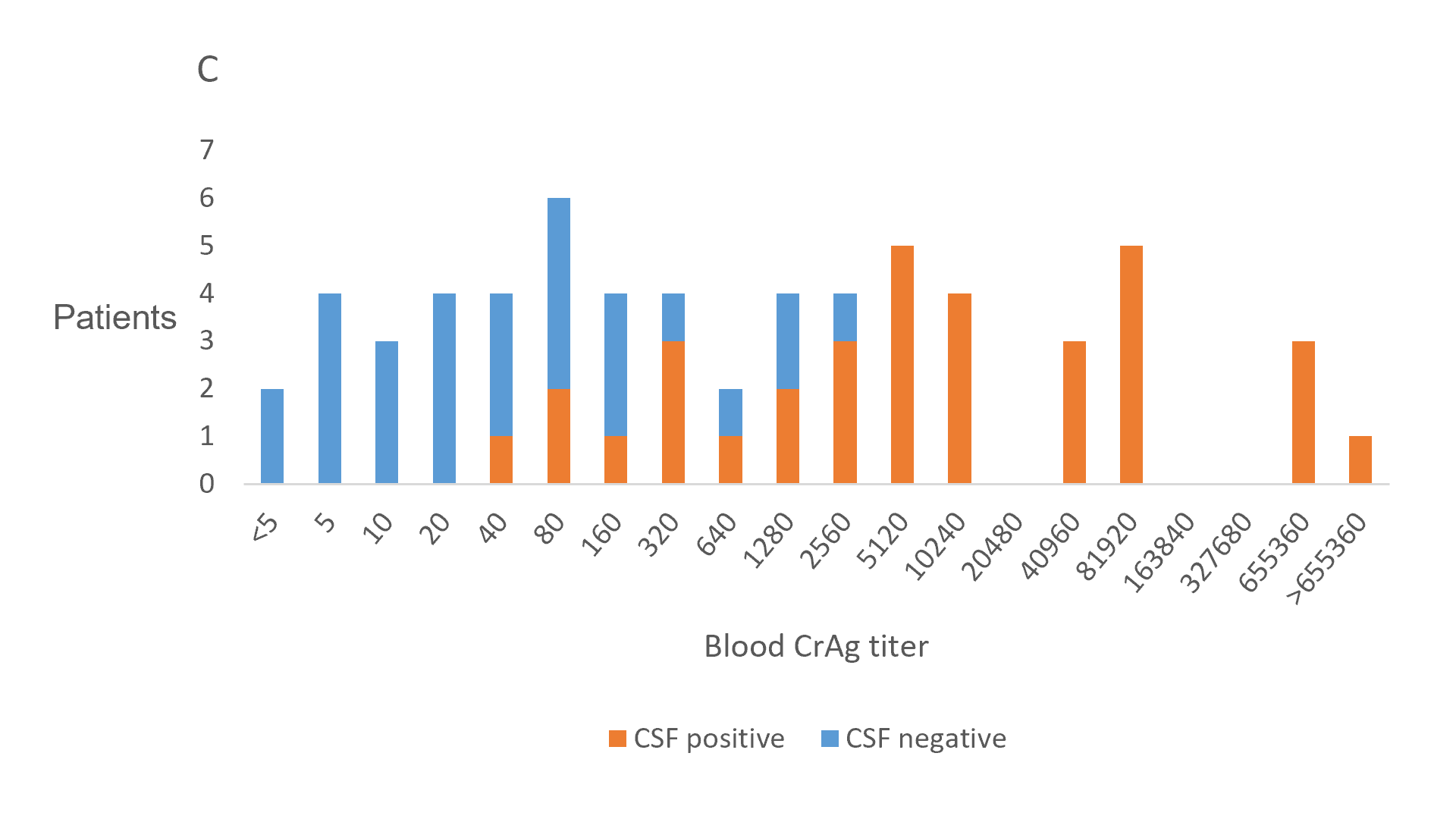
The clinical course of subclinical CM has not been well described. Since higher CrAg titres are known to be associated with a higher risk of death in patients treated with fluconazole monotherapy(2,6,7), it is expected that the prevalence of undiagnosed subclinical CM may be contributing to excess mortality in CrAg screening programmes. However, in a prospective study of CrAg-positive patients, where subclinical CM was identified and treated, there was no increased risk of death associated with CM compared to CrAg-positive patients with negative LPs. Six month mortality was 22/101 (22%)(4), below that observed among CM patients in other comparable non-study settings, indicating that subclinical CM is an earlier or less severe form of disease (Figure 2).
This study will investigate the pathophysiology and clinical outcomes of subclinical CM in order to inform future management strategies, and to increase understanding of host-pathogen interactions in the context of cryptococcosis. Analysing clinical features and parameters of inflammation among individuals with subclinical CM will allow us to delineate if the lack of symptomatic meningism is due to very small quantities of Cryptococcus in the CNS at the time of testing, or whether there are any host or organism-related factors that result in subclinical levels of meningeal inflammation. Following up patients with subclinical CM at the time of CrAg screening, will allow us to assess response to treatment, and estimate survival rates. Comparisons with patients enrolled in the EFFECT study, and surveillance data of similar cohorts of patients with clinical CM treated at the same hospitals in Johannesburg (and CM participants from Tanzania enrolled as part of this study) can then be made. Understanding the pathophysiology and risk of death among patients with subclinical CM will inform future policy regarding the management of patients with asymptomatic cryptococcal antigenaemia. It may, for instance, be adviseable to offer combination antifungal treatment, such as with fluconazole and flucytosine (depending on the results of the EFFECT study), to all patients irrespective of CSF results, if the patient remains asymptomatic. This could potentially avoid hospital admission for LPs and intravenous treatment for this subgroup, reducing financial costs to the health service and opportunity costs to the patient.
Patient Inclusion Criteria
- Consecutive patients aged > 18 yrs
- HIV-seropositive
- CD4 count of <100 cells/μl
- Serum/plasma CrAg test positive
- CSF positive for cryptococcal meningitis (diagnosed by microscopy with India Ink, culture, or CSF CrAg test) at any time since CrAg screening until 1 week following antifungal treatment initiation
- Capacity to provide informed consent for participation, or for symptomatic CM participants in Tanzania, if unable to consent, has a next of kin who agrees to the patient participating
Patient Exclusion Criteria
- Prior episode of CM
- Already taking high-dose fluconazole treatment (800-1200 mg/day) or antifungals for CM (amphotericin-B deoxycholate or liposomal amphotericin-B, and fluconazole or flucytosine) for >1 week
- Clinical symptoms/ signs of meningitis, i.e. severe headache OR headache plus marked nuchal rigidity OR seizures OR Glasgow Coma Scale (GCS) score of <15 at any time since CrAg test (not applicable for recruitment of symptomatic CM participants in Tanzania)
References:
- Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. The Lancet. 2015 May;385(9983):2173–82.
- Wake RM, Govender NP, Omar T, Nel C, Mazanderani AH, Karat AS, et al. Cryptococcal-related Mortality Despite Fluconazole Preemptive Treatment in a Cryptococcal Antigen Screen-and-Treat Program. Clin Infect Dis. 2019 Jun 8;1683–90.
- Longley N, Jarvis JN, Meintjes G, Boulle A, Cross A, Kelly N, et al. Cryptococcal antigen screening in patients initiating ART in South Africa: a prospective cohort study. Clin Infect Dis. 2016 Mar 1;62(5):581–7.
- Wake RM, Britz E, Sriruttan C, Rukasha I, Omar T, Spencer DC, et al. High Cryptococcal Antigen Titers in Blood Are Predictive of Subclinical Cryptococcal Meningitis Among Human Immunodeficiency Virus-Infected Patients. Clin Infect Dis. 2018 Feb 15;66(5):686–92.
- Govender N, Meintjes G, Bicanic T, Dawood H, Harrison TS, Jarvis JN, et al. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. SAJHIVMED. 2013;14(2):76–86.
- Jarvis JN, Percival A, Bauman S, Pelfrey J, Meintjes G, Williams GN, et al. Evaluation of a Novel Point-of-Care Cryptococcal Antigen Test on Serum, Plasma, and Urine From Patients With HIV-Associated Cryptococcal Meningitis. Clin Infect Dis. 2011 Nov 15;53(10):1019–23.
- Rajasingham R, Wake RM, Beyene T, Katende A, Letang E, Boulware DR. Cryptococcal Meningitis Diagnostics and Screening in the Era of Point-of-Care Laboratory Testing. Kraft CS, editor. J Clin Microbiol. 2019 Jan 1;57(1):e01238-18.
- Wake RM, Molloy SF, Jarvis JN, Harrison TS, Govender NP. Cryptococcal Antigenemia in Advanced HIV: Pathophysiology, Epidemiology and Clinical Implications. Clin Infect Dis. 2022 Aug 20;ciac675.